chemistry chapter 6 form 4
NCERT Solutions Class 11 Chemistry Chapter 6 Free PDF Download. The solutions are precise and well structure in simple language for ease of understanding.

Types Of Chemical Reactions Detailed Explanation With Example Videos
Learn vocabulary terms and more with flashcards games and other study tools.
. If 500 mL of a 5 M solution is diluted to 1500 mL what will be the molarity of the solution obtained. E What is the coefficient for carbon dioxide in the balanced equation. Scientific Notation- In which any number can be represented in the form N 10 n Where n is an exponent having positive or negative values and N can vary between 1 to 10.
We can write 232508 as 232508 x10 2 in scientific notation. The 8 electrons of Ni2 sit in five 3d orbitals. Similarly 000016 can be written as 16 x 10 4.
A 2 B 4. Download NCERT Solutions for Class 12 Chemistry Chapter 6. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions.
Here students can access detailed and explanative solutions according to the latest CBSE Syllabus 2022-23 for all the questions present in the NCERT textbook. The number 6022 10 23 called Avogadros number after the 19th-century chemist Amedeo Avogadro is the number we use in chemistry to represent macroscopic amounts of atoms and molecules. Thus if we have 6022 10 23 Oxygen atoms we say we have 1 mol of Oxygen atoms.
6 2 Ni2 electron configuration 8 electrons. 4s23d6---- 4s03d8 Need six orbitals for six ligands but. If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well.
4 C 5 D 6 E 8. In the CBSE Class 10 Science Syllabus 2019-2020 you will find 25 marks allocated to Chemical Substances Nature and Behaviour in. Also students can download the NCERT Solutions for Class 12 Chemistry Chapter 6 from the enclosed link provided below.
4 3d orbitals are full only 1 3d orbital left Must hybridize 1 4s 3 4p and 2 4d to give. Start studying Chemistry Chapter 7-9. It was a relatively complex form of chemistry and stood in stark contrast to inorganic chemistry the principles of which had been established in 1789 by the Frenchman Antoine Lavoisier in his work Traité Élémentaire de Chimie.
Precision refers to the closeness of various measurements for the same quantity. What will be the molarity of a solution which contains 585 g of NaCls per 500 mL. NCERT Solutions for Class 11 Chemistry Chapter 6 Thermodynamics can be found on this page.
A 4 mol L-1 b 20 mol L-1 c 02 mol L-1 d 2 mol L -1. What is the correct form of the conversion factor needed to convert the number of moles of H2O to the number of moles. Similarly you can move ahead by practicing CBSE Class 10 Chemistry Chapter-2 questions or reviewing CBSE Class 10 Chemistry Chapter-2 notes.

Chemistry Form 4 Chapter 2 Structure Of The Atom Notemylife Science Notes Chemistry Notes Study Notes

Selina Concise Chemistry Class 7 Icse Solutions Chapter 6 Metals And Non Metals

Plus Two Chemistry Notes Chapter 6 General Principle And Processes Of Isolation Of Elements Hsslive Guru Plus Two Chemistry Notes Chemistry Notes Chemistry

Multimedia Controlling The Amount Of Products In A Chemical Reaction Chapter 6 Lesson 2 Middle School Chemistry

Selina Concise Chemistry Class 6 Icse Solutions Chapter 4 Elements Compounds Symbols And Formulae Ncert Books Chemistry Class Chemistry Chemistry Classroom

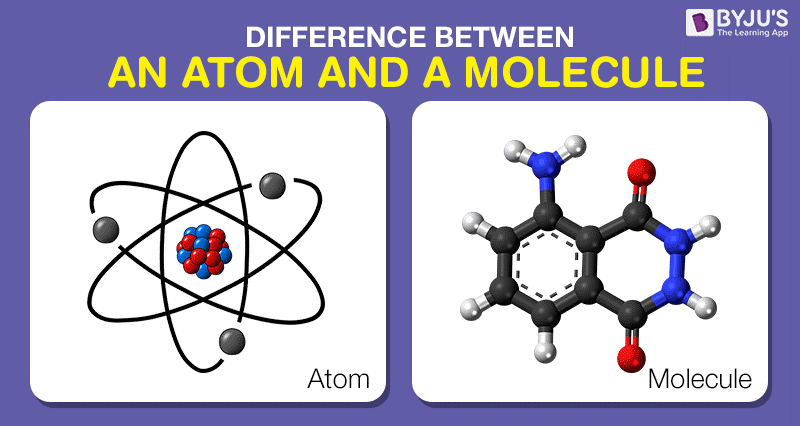
Difference Between Atom And Molecule In Tabular Form

Selina Concise Chemistry Class 8 Icse Solutions Chapter 6 Chemical Reactions Cbse Tuts Cbsetuts Selinaconcisechemistrycla Chemistry Class Chemistry Class 8

Melting And Freezing Point Of Naphthalene Chemistry Notes School Study Tips Study Notes

Energy Heat And Work In Chemistry Teaching Chemistry Chemistry Classroom Chemistry

Ncert Solutions For Class 12 Chemistry Chapter 6 General Principles And Processes Of Isolation Of Elements Chemistry Lessons Chemistry Chemistry Experiments

Electronegativity Definition Periodic Trends Effect On Bonding Faqs

Diffusion Chapter 2 Form 4 Chemistry Notes Chemistry Education Bible Study Lessons

Diffusion Chapter 2 Form 4 Chemistry Notes Chemistry Education Bible Study Lessons

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 2 Acids Bases And Salts Free Pdf

Multimedia Carbon Dioxide Can Make A Solution Acidic Chapter 6 Lesson 10 Middle School Chemistry

Selina Solutions Class 9 Concise Chemistry Chapter 4 Atomic Structure And Chemical Bonding Download Free Pdf

Properties Of Acids And Bases Physical And Chemical Properties With Examples

Comments
Post a Comment